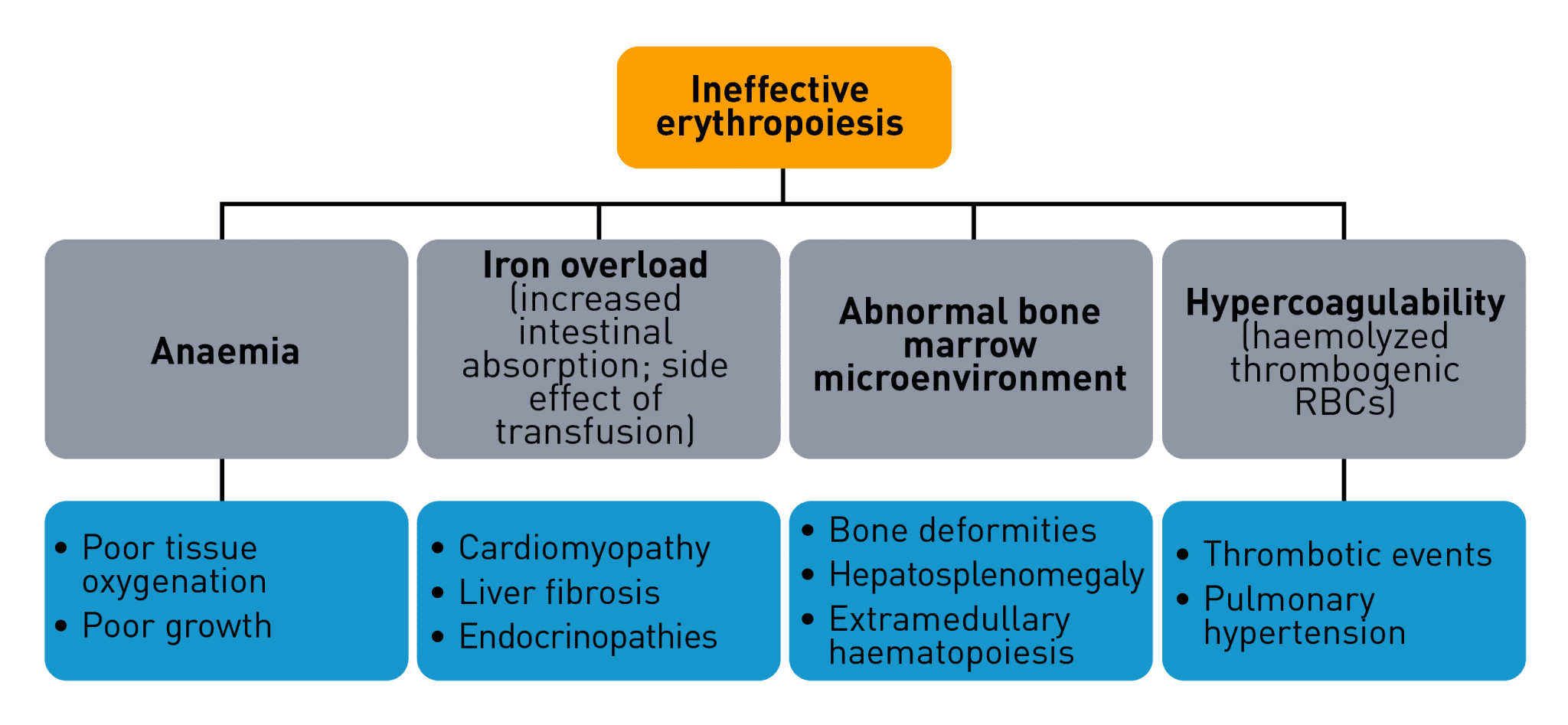
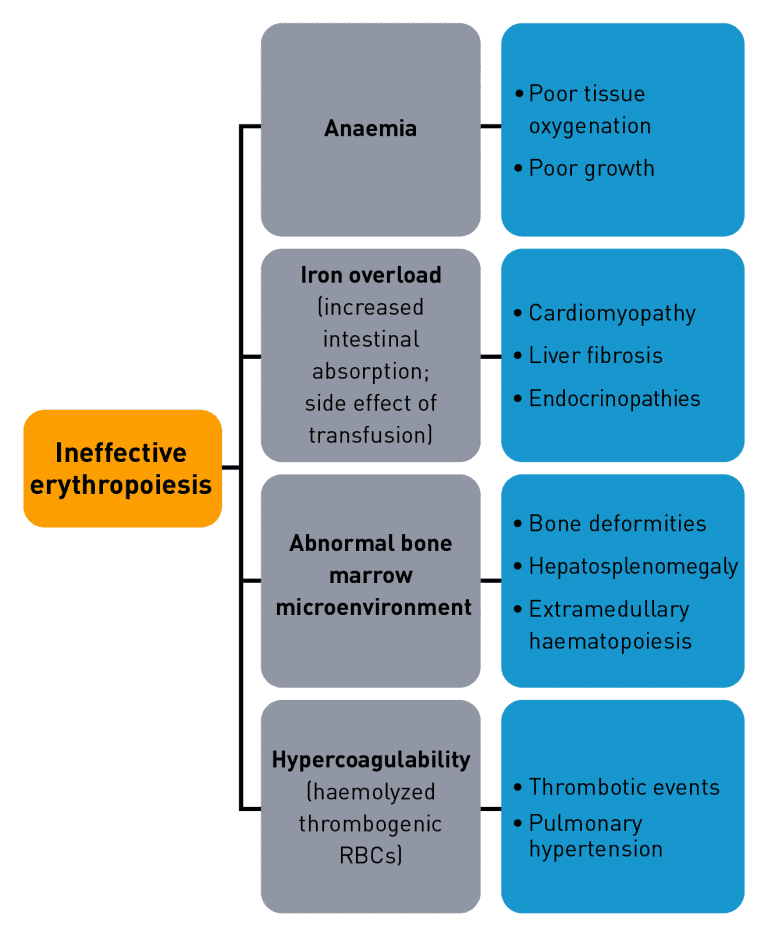
Chronic anaemia and ineffective erythropoiesis
Anaemia, a condition that affects almost 25% of the global population, refers to the shortage of functional haemoglobin or reduction in red blood cells.1,2 Anaemia reduces the delivery of oxygen to tissues, which is compensated primarily by increasing cardiac output; the resulting tissue hypoxia can affect the function of major organs.3 Chronic, severe anaemia is associated with increased risk of death due to cardiac failure.4,5 Reduced delivery of oxygen to tissues also causes other symptoms such as fatigue, dyspnoea, tachycardia, hypotension, low body temperature and enlarged spleen.6
The main cause of anaemia is iron deficiency, closely followed by anaemia associated with chronic disease.6 This website will discuss and focus on anaemia associated with haematological diseases.
In anaemia associated with chronic haematological diseases, reduced red blood cell production may occur due to ineffective erythropoiesis. Ineffective erythropoiesis causes an imbalance in the various progenitor and precursor cells that proliferate, differentiate and mature into red blood cells, leading to impaired erythropoiesis.7-11 Ineffective erythropoiesis is a hallmark of certain haematological diseases such as:7-10,12-16
- myelodysplastic syndromes
- thalassaemias (e.g. specific types of α- and β-thalassaemia)
- aplastic anaemia
- myelofibrosis
- sickle cell anaemia
- congenital dyserythropoietic anaemias
- inherited sideroblastic anaemias.
Please see Chronic anaemia in haematological diseases for further details
Early- versus late-stage erythropoiesis
Early-stage erythropoiesis is characterised by proliferation of early-stage erythroid cells (progenitors), whereas late-stage erythropoiesis involves the differentiation and maturation of late-stage erythroid cells (precursors).18
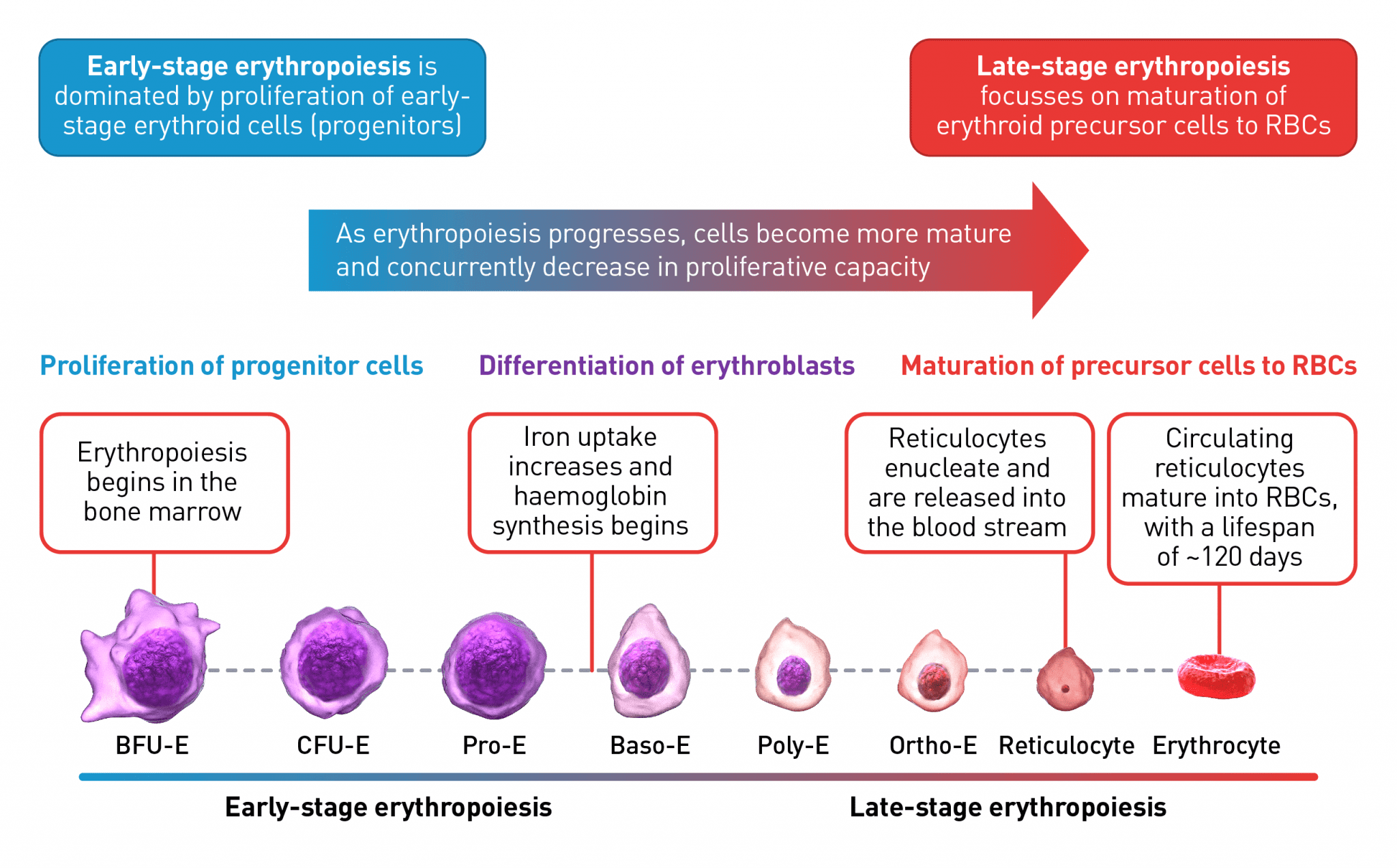
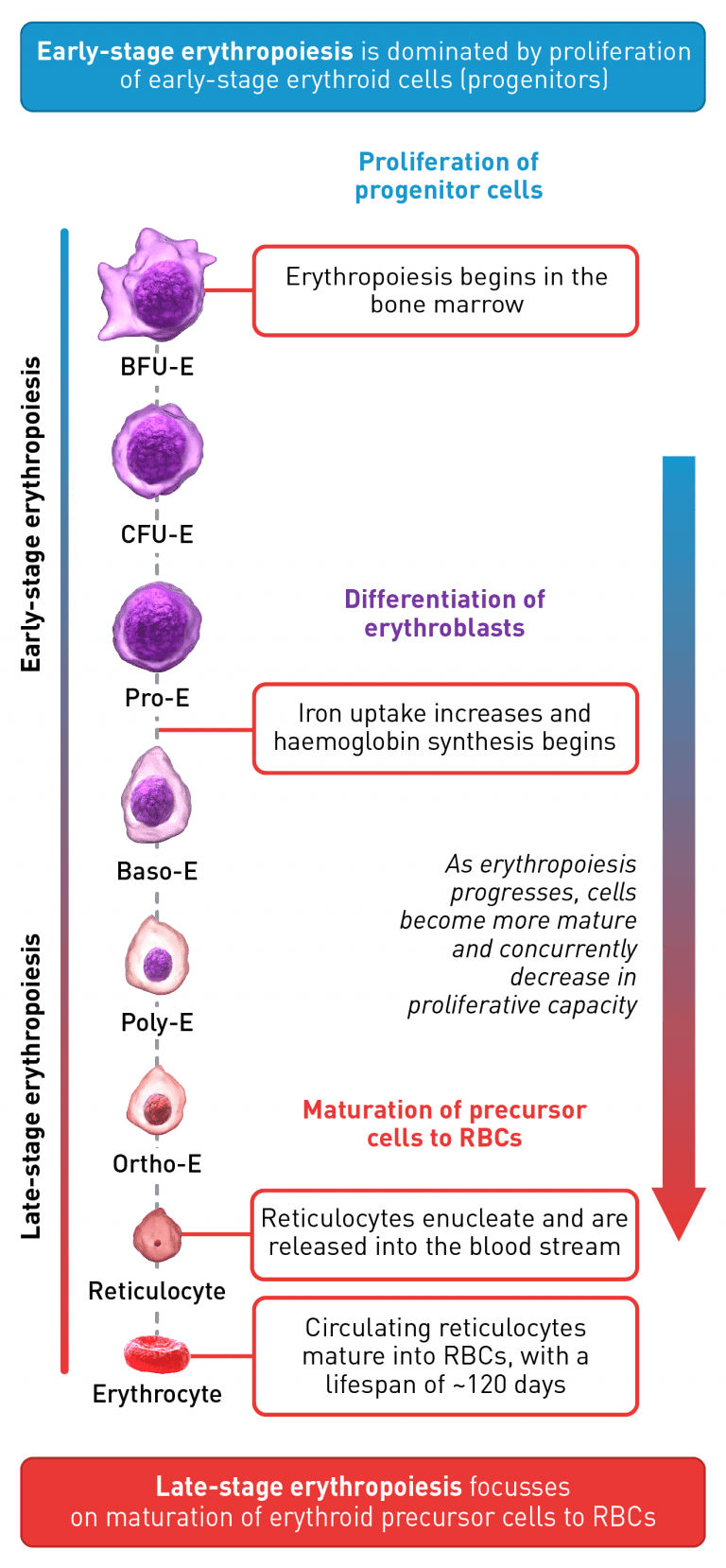
The erythroid progenitor cell compartment contains the early erythroid progenitors. BFU-E represent the earliest progenitors committed exclusively to erythroid maturation, which differentiate into late CFU-E and proerythroblasts.19 The earliest recognisable erythroid cells are the Pro-E, which undergo morphological changes such as reduction in cell size, protein production including haemoglobin, and reduction in proliferative capacity, evolving through the erythroblast stages, Baso-E, Poly-E and Ortho-E, successively.20,21 At the end of the terminal maturation, erythroblasts expel their nuclei and lose all their organelles resulting in mature enucleated cells, called reticulocytes.20 After expelling its nucleus, the reticulocyte is released into the bloodstream where maturation continues in order to produce fully functional, biconcave erythrocytes within 1–2 days.20
Baso-E, basophilic erythroid; BFU-E, burst-forming unit-erythroid; CFU-E, colony-forming unit-erythroid; Ortho-E, orthochromatophilic; Poly-E, polychromatophilic; Pro-E, proerythroblasts; RBC, red blood cell.
The process is tightly regulated by many different transcription factors and signalling pathways, as shown in the figure below.13,18,22-30
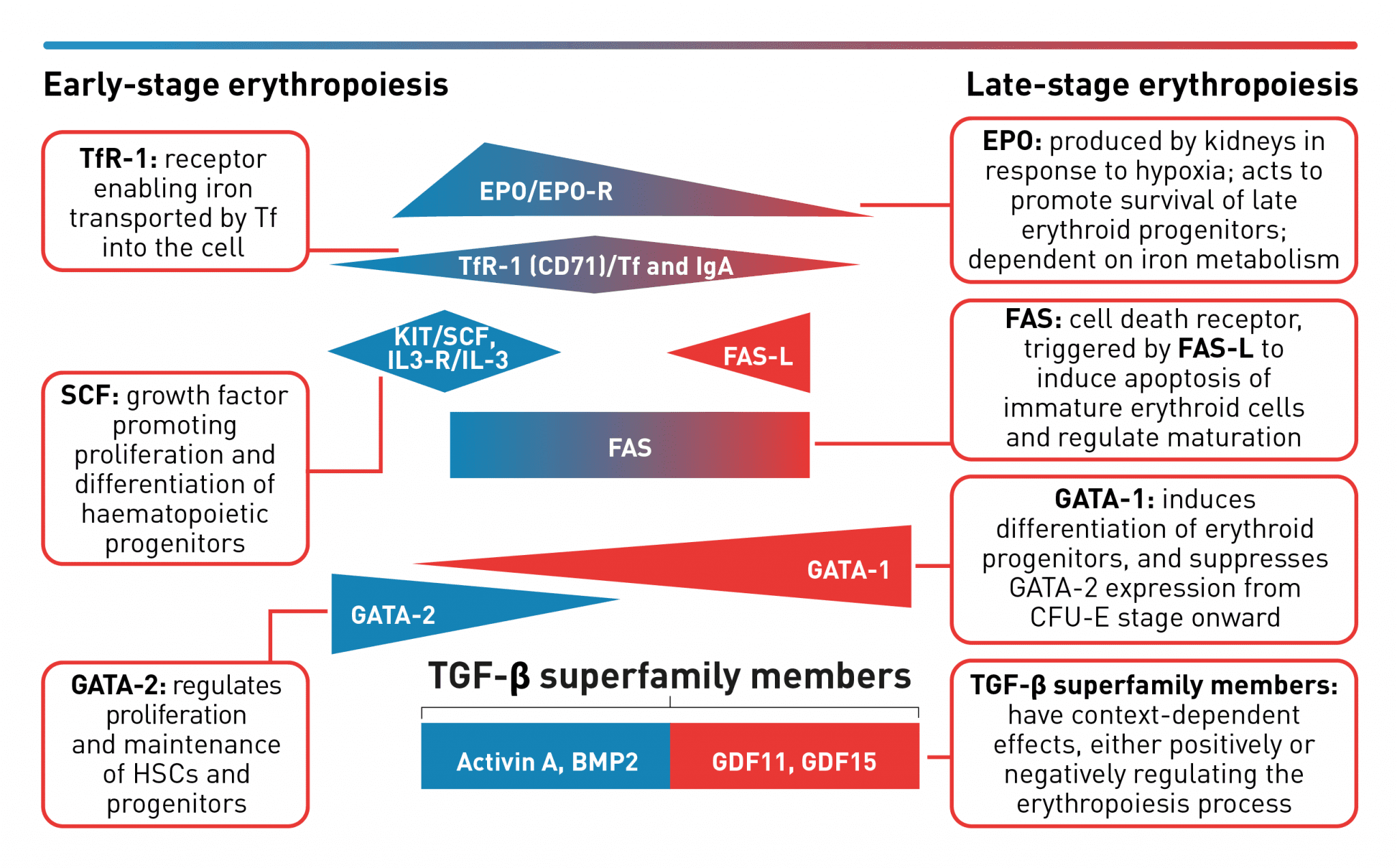
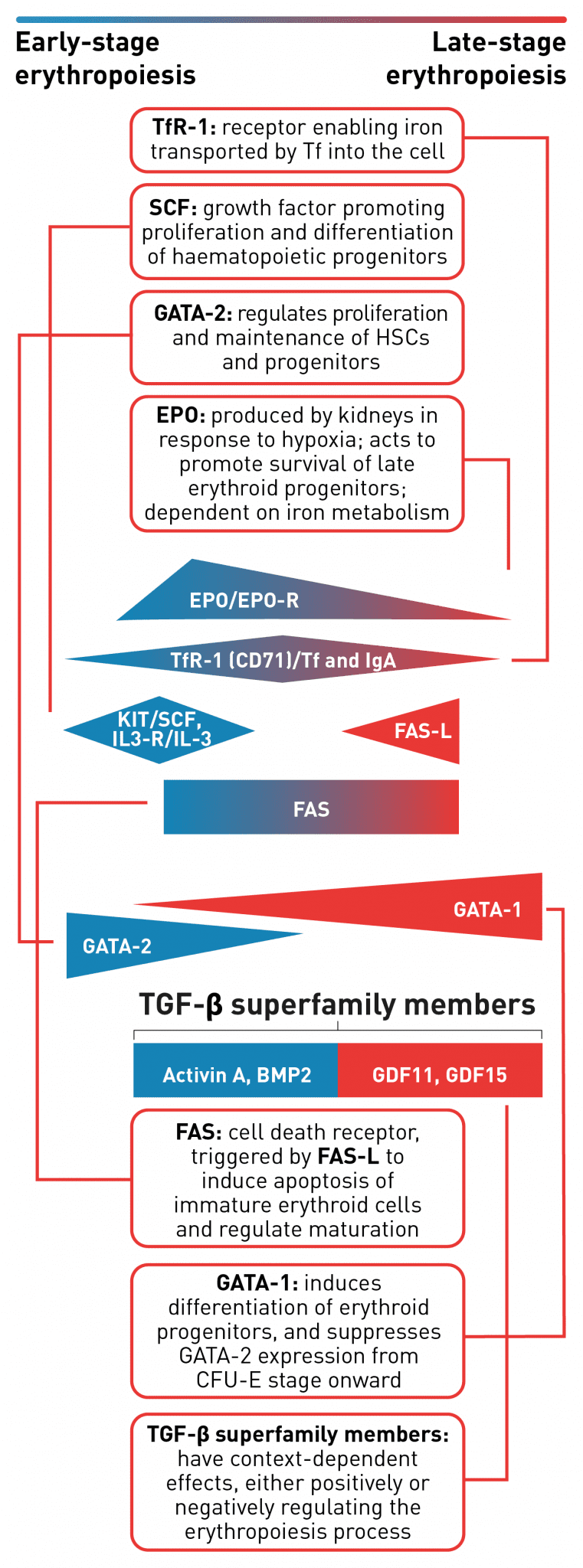
A complex network of transcription factors and epigenetic regulators ensure appropriate numbers of red blood cells are produced.18,31,32 EPO promotes survival and proliferation of erythroid progenitor cells by preventing apoptosis.33 GATA-1 is the main regulator of lineage-commitment, differentiation and survival of early-stage erythroid cells18,31 Other transcription factors include regulators of iron metabolism, such as TfR1 and early acting haematopoietic growth factors such as SCF.18 FAS-L is expressed by late-stage erythroid cells to trigger apoptosis of immature erythroid cells and regulate maturation, while selected TGF-β superfamily ligands also play a key role in regulating erythroid maturation.18,30,32,34,35 Tight regulation of erythropoiesis is crucial to ensure a steady-state process.18
BMP2, bone morphogenetic protein 2; EPO, erythropoietin; CFU-E, colony-forming unit-erythroid; EPO-R, erythropoietin receptor; FAS-L, FAS ligand; GDF, growth differentiation factor; HSC, haematopoietic stem cells; IL-3, interleukin 3; IL-3-R, interleukin 3 receptor; IgA, immunoglobulin A; SCF, stem cell factor; Tf, transferrin; TfR-1 (or CD71), transferrin receptor; TGF, transforming growth factor.
Erythropoietin stimulates red blood cell production by acting as a key positive regulator of early-stage erythropoiesis; its greatest influence occurs during the colony-forming unit-erythroid (CFU-E)/proerythroblasts stage of erythropoiesis and wanes during the later maturation stages.13,33,36,37 The transcription factor GATA-2 is highly expressed in haematopoietic stem cells and early-stage progenitors, but its expression is suppressed from the CFU-E stage and beyond.38 Expression of GATA-1 is first seen in CFU-E progenitors and increases as the erythroblasts differentiate and mature. This transition of GATA expression from GATA-2 to GATA-1 during the erythropoiesis process, as indicated in the figure above, is referred to as the GATA switch mechanism, and results in the mutually exclusive expression of GATA-2 and GATA-1 during early and late stages of erythropoiesis, respectively.23,38, FAS ligand is expressed by late-stage erythroid precursor cells and is involved in regulating the final maturation stages that lead to the generation of functional red blood cells.18 Members of the transforming growth factor β (TGF-β) superfamily can exert opposing regulatory effects on the erythropoiesis process.30 Signalling mediated by selected TGF-β superfamily ligands regulates erythroid maturation via the Smad pathway; dysregulation of this signalling pathway may contribute to impaired erythroid maturation leading to ineffective erythropoiesis.34,39,40
Characteristics of ineffective erythropoiesis
Haematological disorders are often associated with alterations in certain signalling pathways that regulate erythropoiesis.8,9,11,34,39,41-43
Please see Erythroid maturation defect (EMD) for further details
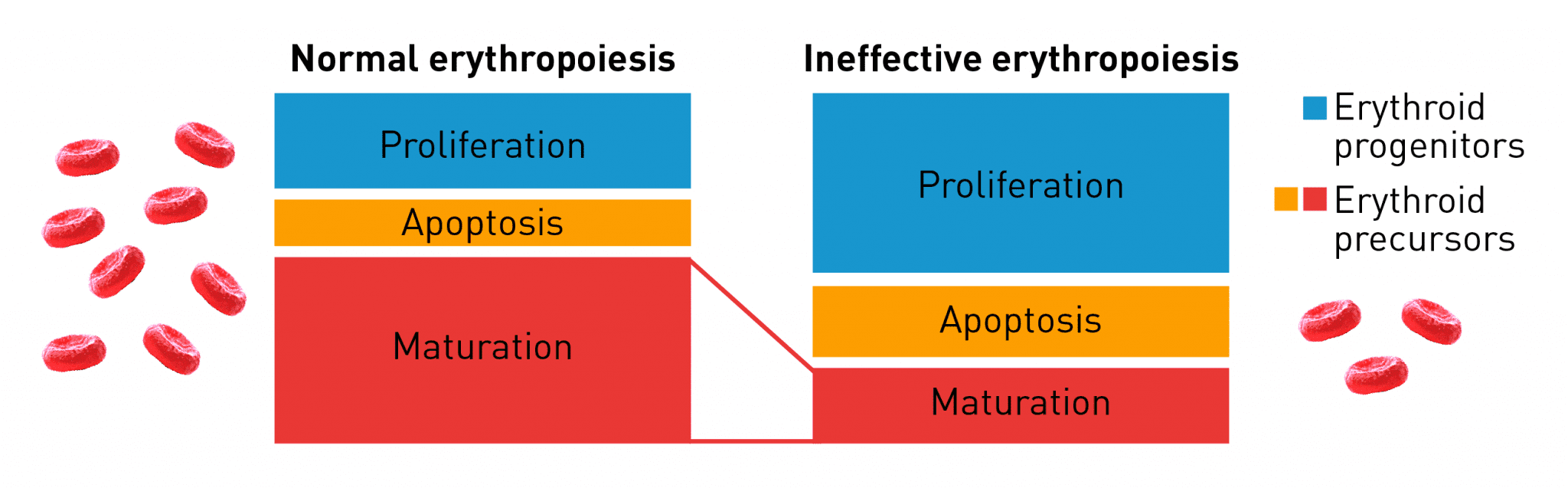
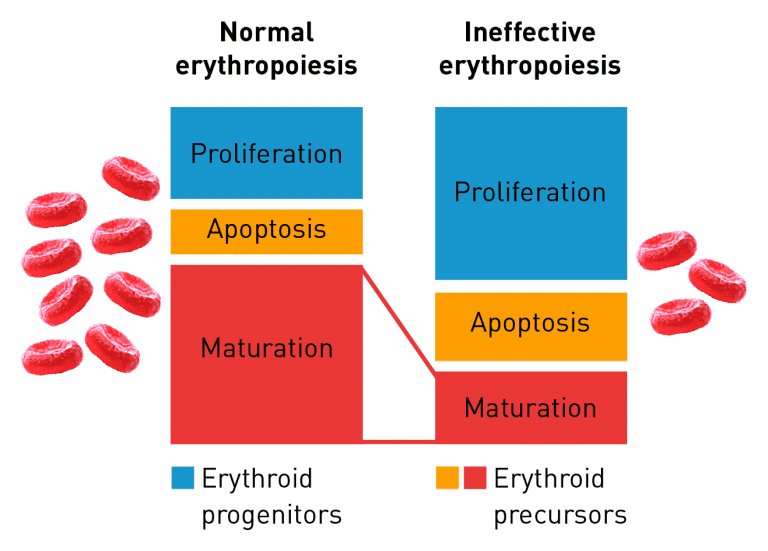
Normal erythropoiesis proceeds with maturation and apoptosis tightly regulated to maintain red blood cell homeostasis and normal oxygen levels. Ineffective erythropoiesis is an ongoing pathological state in which increased erythroid proliferation cannot restore red blood cell count due to increased apoptosis of immature erythroid cells and impaired late-stage maturation.11