Myelodysplastic syndromes (MDS)
MDS can be grouped into two major categories: higher risk or lower risk.6 Patients with higher-risk MDS carry a major risk of progression to acute myeloid leukaemia and short survival.7 Lower-risk MDS account for the majority of patients with MDS with anaemia being the main clinical challenge.6,8
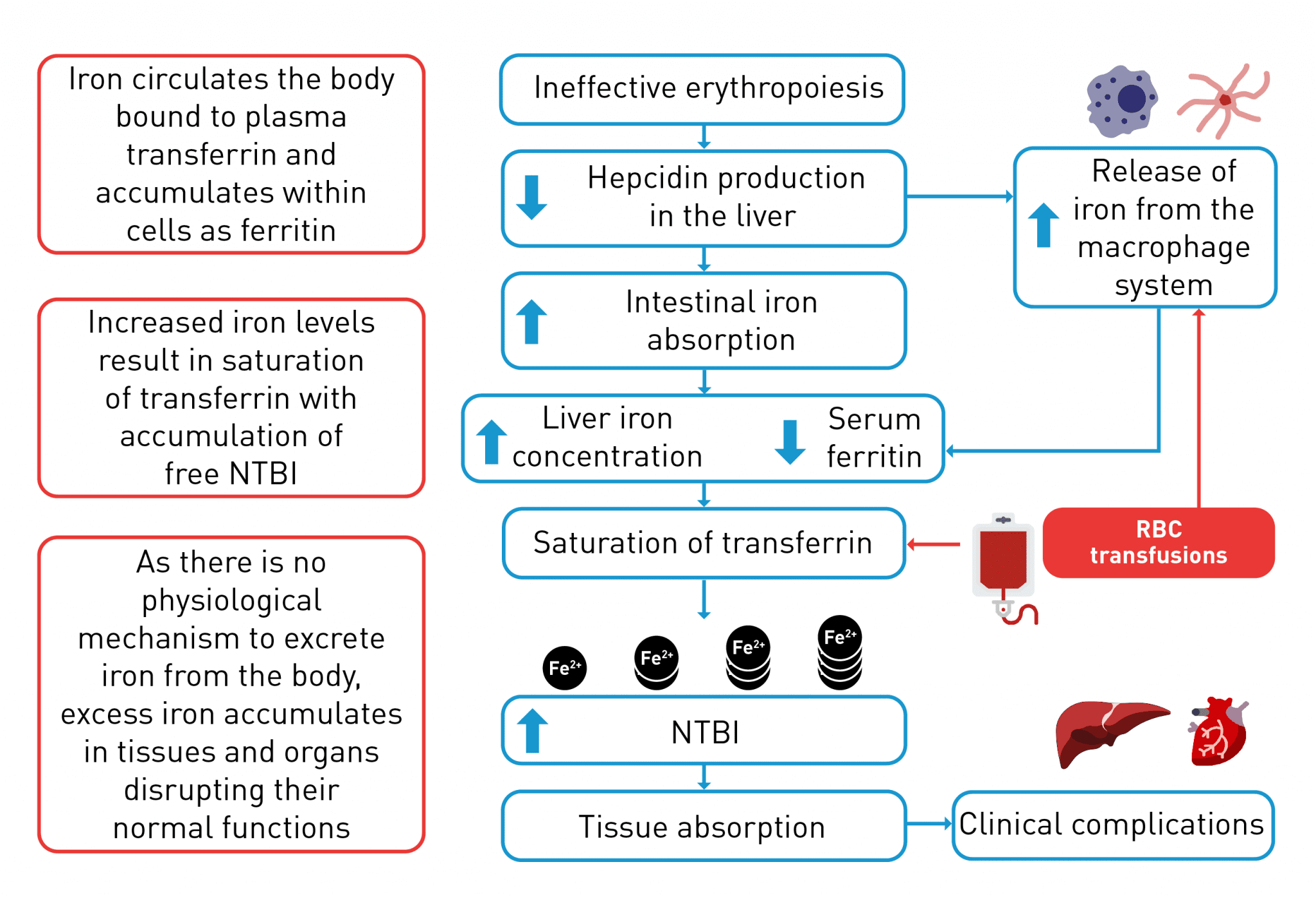
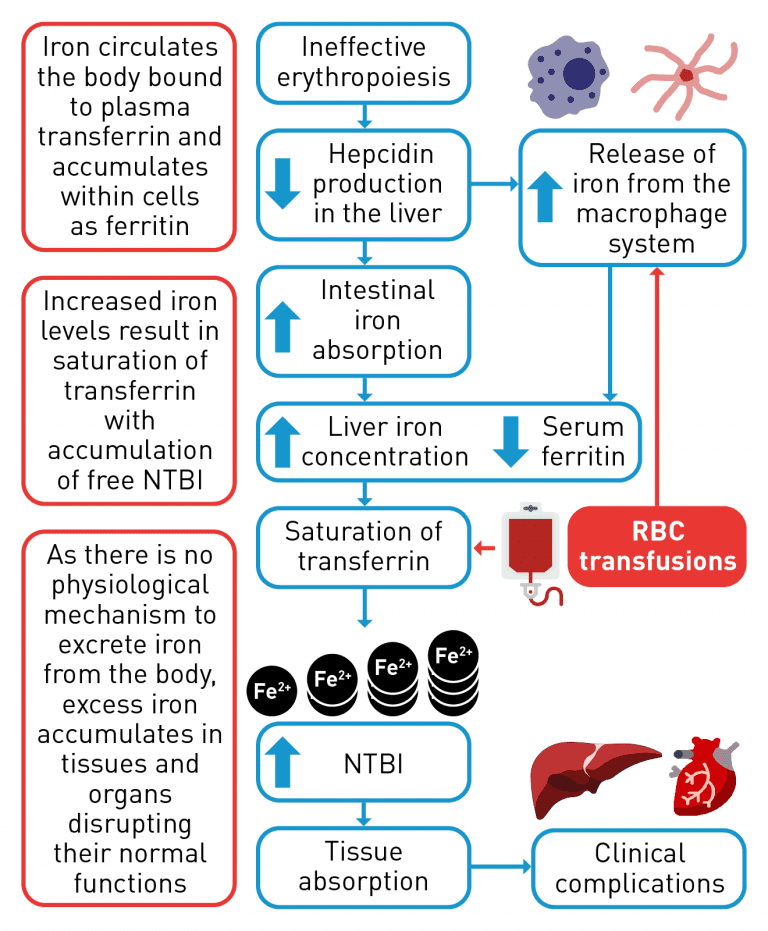
Red blood cell transfusions provide temporary management of severe anaemia, as patients who receive transfusions may continue to experience fluctuations in haemoglobin.9 Patients receiving red blood cell transfusions may also experience complications, such as iron overload and alloimmunisation.10,11
Other haematological diseases
Aplastic anaemia
The goals of treatment for aplastic anaemia are to restore haematopoietic stem cells and ameliorate cytopenia-related complications.18 Treatment options may include stem cell transplant (the only treatment to offer a potential cure), immunosuppressive therapy, and stem cell stimulation therapy may be an option for patients refractory to or relapsing after immunosuppressive therapy and androgens.18-20 Blood transfusions (of platelets or red blood cells) and other supportive care measures are often utilised.18
Myelofibrosis
The current treatment goals for myelofibrosis are to reduce the burden of symptoms and reduce the risk of leukaemic transformation.21,22 For high-risk patients, treatment options may include stem cell transplant (the only treatment capable of extending survival or offering a potential cure) or an investigational agent within a clinical trial.21,22 For low-risk patients, observation alone may be an option and for intermediate-risk patients, and low-risk patients requiring treatment, options include investigational agents in the setting of a clinical trial.21 For patients not eligible for stem cell transplant or clinical trial enrolment, symptom-directed therapeutic options include conventional treatments for anaemia, splenomegaly, constitutional symptoms, localised bone pain, or symptomatic extramedullary haematopoiesis.21,22
Sickle cell anaemia
Treatment for sickle cell anaemia usually aims to relieve symptoms and prevent complications.23 Treatment may include red blood cell transfusions, antibiotics to treat infections, antibiotics as prophylaxis to prevent infections in infants, pain relieving medications and hydroxyurea to reduce the frequency of painful crises.23,24 Stem cell transplant is an option to provide a potential cure but is usually only offered to younger patients.23
Congenital dyserythropoietic anaemias
Congenital dyserythropoietic anaemias are generally managed using red blood cell transfusions and iron chelation therapy. As with β‑thalassaemia, the only definitive cure for patients with congenital dyserythropoietic anaemias is stem cell transplant, however, this is limited to patients with very severe anaemias.3,25
Inherited
sideroblastic
anaemias
For mild inherited sideroblastic anaemias a watch and wait approach may be adopted whilst patients with mild-to-severe anaemia may be treated with ESAs or regular red blood cell transfusions.2 Hypomethylating agents and stem cell transplant may be considered for the most severe cases.2
The unmet need in the treatment of chronic anaemia
For many haematological diseases, the only potential definitive cure is stem cell transplant, which is limited to a small number of eligible patients. Gene therapy may be potentially curative for patients with β-thalassaemia, but as with stem cell transplant, gene therapy is only appropriate for a specific subset of patients.3,7,15,23 Other treatments, such as ESAs and red blood cell transfusions with iron chelation therapy have limitations due to their own complications.1,4,11 ESAs can be effective in patients without adverse prognostic factors, however, many patients with lower-risk MDS receiving ESAs either relapse after an initial response or have primary resistance to these agents; for these patients there are limited effective treatment options available after the failure of ESAs.41 Red blood cell transfusions may result in iron overload and other complications, whilst iron chelation therapy poses certain challenges such as poor adherence and regular monitoring.14,33,37,40 Therefore, in haematological diseases there is a significant unmet need for therapies targeting alternative pathways involved in the development of chronic anaemia, such as those implicated in ineffective erythropoiesis caused by an erythroid maturation defect, with the goal of reducing patient reliance on red blood cell transfusions and iron chelation therapy.
Please see Erythroid maturation defect (EMD) for further details